electron configuration of ru2+|Complete Electron Configuration of Ruthenium (Ru, Ru3+) : Bacolod Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa Wan Hai Lines (Phils.), Inc. is a Philippines company, located on Philippines, Its address is 18th Floor, Rufino Pacific Tower, #6784 Ayala Avenue Corner V.A. Rufino Street, 1223, Makati City, Metro Manila, Philippines, Makati .
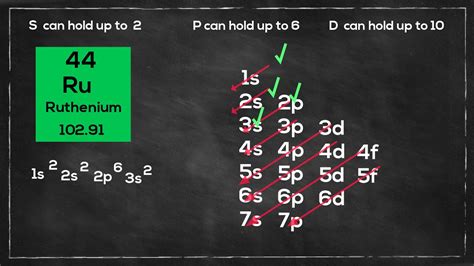
electron configuration of ru2+,The ground state electron configuration of ruthenium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d7 5s1. This electron configuration shows that the last shell of ruthenium has an electron and the d-orbital has a total of seven electrons. Therefore, the valence electronsof ruthenium are eight. The elements . Tingnan ang higit pa
electron configuration of ru2+The total number of electrons in ruthenium is forty-four. These electrons are arranged according to specific rules in different . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit paAgo 14, 2018 — Electron configuration Ruthenium Ruthenium has an atomic number of 44 and has 44 electrons. It is an exception to the normal rules of electron configuration because instead of having .Ruthenium is a transition metal with atomic number 44. The electron configuration of ruthenium is 1s2 2s2p6 3s2p6d10 4s2p6d7 5s1. Ruthenium has a hexagonal crystal .Set 4, 2014 — You've already figured out that the electron configuration of $\ce{Ru}$ is $\ce{[Kr]\,4d^7\,5s^1}$. All you need to know know is the order of energies for the .
Nob 13, 2020 — Electron configuration of Ruthenium is [Kr] 4d7 5s1. Possible oxidation states are +3. Electron Configuration. The periodic table is a tabular display of the .

Hun 27, 2024 — This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, .
Orbital diagram. Ruthenium electron configuration. ← Electronic configurations of elements. Ru (Ruthenium) is an element with position number 44 in the periodic table. .The simplified electron configuration of ruthenium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d7 5s1. Adding all these indices, the total is 44, which is the total number of electrons it has, which is equal to its atomic number. .The electron configuration of ruthenium is 1s2 2s2p6 3s2p6d10 4s2p6d7 5s1. This arrangement of electrons illustrates how they are distributed within the atomic orbitals. In .Give the ground state electron configuration and number of unpaired electrons in a Ru2+ ion. (LO 6.1, 6.2) (a) 3Kr45s2 4d4 0 unpaired electrons (b) 3Kr45s2 4d6 0 unpaired .Question: Predict the ground-state electron configuration of each ion. Use the abbreviated noble gas notation. . Ru2+: [Kr]4d6 [Xej4f14 503 . Show transcribed image text. There are 3 steps to solve this one. Step 1. Given: Ru A 2 + W A 3 + View the full answer. Step 2. Unlock. Step 3. Unlock. Answer. Unlock. Previous question Next question .The electron configuration notation for ruthenium is [Kr].4d75s1, indicating the distribution of electrons in each energy level and orbital. This notation follows the rules of electron filling, where the noble gas krypton represents the filled energy levels up to that point, and the remaining electrons are added to the respective orbitals.The order of filling the orbitals with electrons in the Ru atom is an exception to the rule. Expected electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 6 But in reality, one electron moves from the 5s orbital to the 4d orbital: Electronic configuration of the Ruthenium atom in ascending order of orbital energies:_____ The first 36 electrons have filled out the 1s, 2s, 2p, 3s, 3p, 4s, 3d and 4p sublevels. The remaining \Box electrons of ruthenium's configuration need to be written out. Thus, the remaining \Box electrons fill the _____ orbitals. sing the maximum number of electrons that can fill each orbital, write out the electron configuration.
Okt 29, 2021 — Ru2+ has one more electron than Ru3+. So there should be total of 6 electrons in the orbitals. Although you expect Ru2+ to be [Kr] 4d^4, 5s^2, the reason it is [Kr] 4d^5, 5s^1 is because the atom is most stable when the .
Mar 23, 2023 — Shorthand Electron Configuration Full Electron Configuration Electron shell arrangement; 1: Electron configuration of Hydrogen (H) 1s 1: 1s 1: 1: 2: Electron configuration of Helium (He) 1s 2: 1s 2: 2: 3: Electron configuration of Lithium (Li) [He] 2s 1: 1s 2 2s 1: 2, 1: 4: Electron configuration of Beryllium (Be) [He] 2s 2: 1s 2 2s 2: 2, 2: 5 .
Mar 24, 2016 — DOI: 10.1039/C6RA02558G Corpus ID: 101597372; Formation of a robust Ru4O4 skeleton with two Ru2(CO)4 units in criss–cross configuration @article{Yang2016FormationOA, title={Formation of a robust Ru4O4 skeleton with two Ru2(CO)4 units in criss–cross configuration}, author={Jindou Yang and Wang Xian .Ago 14, 2018 — Electron configuration RutheniumRuthenium has an atomic number of 44 and has 44 electrons.It is an exception to the normal rules of electron configuration be.Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .In the case of Ru 2 + {^{2+}} 2 + ion, we have to detract two electrons from the previous electron configuration (because of the double positive charge), thus, the new electron configuration will be as following: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 4 d 6 \mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6 4d^6} 1 s 2 2 s 2 2 p 6 3 s .Mar 30, 2020 — Cr and Cu have unique configurations because they steal electrons from the 4s because they prefer to be half-full or full orbitals. So if you have 4 electrons in the 3d, like in Cr, it is more energetically .
Question: Write the electronic configuration for each of the following: a. Mn2+, Ru2+, Rh2+ b. How many unpaired electrons are in the d orbitals of an octahedral complex of Mn2+ , assuming a strong-field complex? c. How many unpaired electrons are in the d orbitals of an octahedral complex of Ru2+ , assuming a strong-field complex? d.

In order to obtain the electron configuration of Ru 2 + ^{2+} 2 +, remove one electron from the 5s orbital and one electron from the 4d orbital: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 4 d 6 \boxed{1s^22s^22p^63s^23p^64s^23d^{10}4p^64d^6} 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 4 d 6The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell.
electron configuration of ru2+ Complete Electron Configuration of Ruthenium (Ru, Ru3+)The ruthenium atom belongs to the transition metal group with an electron configuration of [Kr] 4 d 7 5 s 1 4d^75s^1 4 d 7 5 s 1. However, as we can see from the charge on Ru, the ruthenium has lost two electrons; thus, its electron configuration will be: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 4 d 6 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d .Complete Electron Configuration of Ruthenium (Ru, Ru3+)NOTE: Copper is an exception to the rules for writing electron configurations! Video: Cu, Cu +, and Cu 2+ Electron Configuration Notation In writing the electron configuration for Copper the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital.
May 16, 2021 — About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .
Mar 18, 2023 — Therefore, the electron configuration of nickel(Ni*) in an excited state will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d xy 2 3d yz 2 3d zx 2 3d x 2-y 2 1 3d z 2 1 4s 1 4p x 1. The valency of the element is determined by electron configuration in the excited state. Here, nickel has four unpaired electrons. So, in this case the valency of nickel is 4 .
electron configuration of ru2+|Complete Electron Configuration of Ruthenium (Ru, Ru3+)
PH0 · What would the Electronic Configuration of Ru²⁺ be?
PH1 · Ruthenium
PH2 · Give the ground state electron configuration and number of unpair
PH3 · Electron configuration of Ruthenium
PH4 · Electron configuration for Ruthenium (element 44). Orbital diagram
PH5 · Electron Configuration for Ruthenium
PH6 · Electron Configuration Ruthenium (Electron configuration
PH7 · Electron Configuration For Ru
PH8 · Electron Configuration Calculator
PH9 · Complete Electron Configuration of Ruthenium (Ru, Ru3+)